Policies & Guidelines
- Double Blind Peer Review Process & Editorial Process
- Publication Frequency
- Open Access Policy
- Archiving
- Crossmark Policy Statement
- Publication Ethics and Policy Guidelines
- Plagiarism Policy
- Policies Related to the Use of AI in Manuscripts
- Repository Policy
- Data Sharing Policy
- Advertisement Policy
- Appeals Process
- Complaint Process
- Submission of False Documents
1.DOUBLE BLIND PEER REVIEW PROCESS & EDITORIAL PROCESS
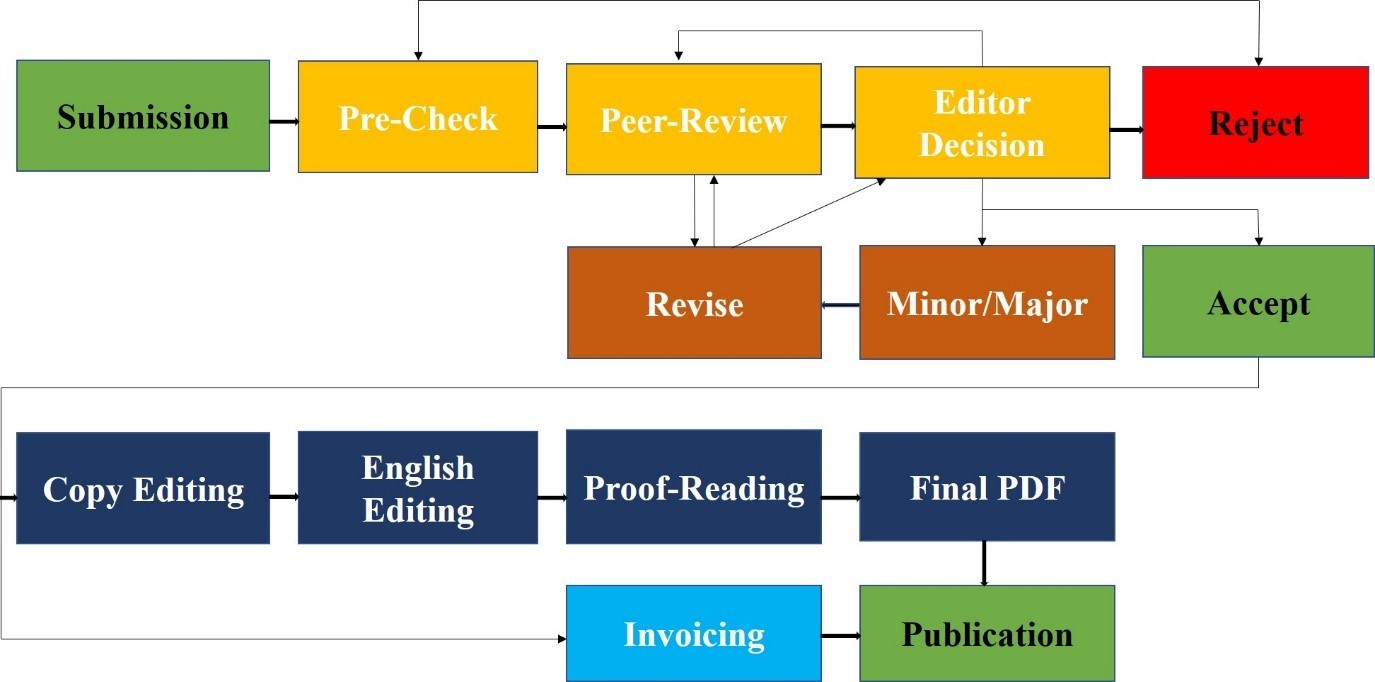
This Policy is applicable to all publications submitted to the research journals of Lahore Medical Research Center (LMRC) and operates a double-blind peer review process. The Editorial Board of the Journal is responsible for the selection of reviewers based on their expertise in the relevant field. The manuscript is sent to two external reviewers (from outside the organization of journal) for a peer review. The entire publication process completes in a range of 40-45 days. In case of conflict of interest regarding a specific manuscript, a member of the Editorial Board will be assigned to assume responsibility for overseeing peer review. Reviewers will be treated anonymously and the pre-publication history of each article will not be made available online. Intentionally falsifying information, for example, authors or reviewers with a false name or email address, will result in rejection of the manuscript and may lead to penalty according to misconduct policy. Guest editorial submitted by authors is reviewed internally by Editor-in-Chief or Editor and is then sent externally to another guest editor for final verdict.
Use of Generative AI and AI-Assisted Technologies in the Peer Review Process
a. Journal upholds the highest standards of integrity and confidentiality in the peer review process. Manuscripts sent for review are strictly confidential documents and must not be shared, uploaded, or processed using generative AI or AI-assisted tools. Doing so may compromise the confidentiality and proprietary rights of authors, and, in cases involving personal data, may also breach data privacy regulations.
b. This confidentiality requirement also applies to peer review reports, which may include sensitive or identifiable information. Reviewers should therefore not use AI tools—even for tasks such as improving language or readability—when preparing their review reports.
c. Peer review is a human-driven process that requires independent judgment, critical evaluation, and subject expertise. Generative AI or AI-assisted technologies must not be used to perform or assist in the scientific evaluation of manuscripts. Such technologies lack the nuanced reasoning and accountability expected of human reviewers and may introduce errors, bias, or incomplete analysis.
2. PUBLICATION FREQUENCY
Monthly
3. OPEN ACCESS POLICY
All articles in PJHSL are open access and freely available online. The journal's contents are released under the Creative Commons Attribution licence, which allows for unrestricted use, distribution, and reproduction in any medium as long as the original authors and source are cited.
4. ARCHIVING
Long-term preservation and archiving (LPTA) of data ensures that the journal’s data is backed up in a network of libraries created by a dedicated digital archiving and preservation service. PJHSL employs PKP Private’s LOCKSS network to create a file distributed among the participated libraries, allowing these libraries to create permanent files of the journal with goals to preserve and restore the digital data. Other than that, a separate doi has been assigned to each article.
5. CROSSMARK POLICY STATEMENT
DOI: 10.54393/pjhs/crossmark
All articles published in Pakistan Journal of Health Sciences-Lahore (PJHSL) receive a DOI and are permanently published. This applies regardless of the outcome of the peer review that follows after publication.
Authors can revise, change and update their articles by publishing new versions, which are added to the article’s history; however, the individual versions, once published, cannot be altered or withdrawn and are permanently available on the Pakistan Journal of Health Sciences-Lahore (PJHSL) website. Pakistan Journal of Health Sciences-Lahore (PJHSL) participates in the CrossMark scheme, a multi-publisher initiative that has developed a standard way for readers to locate the current version of an article. By applying the CrossMark policies, Pakistan Journal of Health Sciences- Lahore (PJHSL) is committed to maintaining the content it publishes and to alerting readers to changes if and when they occur.
Addendum
If critical results were accidentally omitted from the original publication, an Addendum may be submitted to include the missing information. This Addendum will be published in the current issue of the journal and hyperlinked to the original publication, without requiring an update to the original paper.
Erratum
An Erratum should be published for scientifically relevant formatting changes or errors in the author or contributor list. Minor errors, such as spelling or grammatical mistakes that do not impact the meaning or readability of the article, do not qualify for an Erratum. All authors should carefully proofread the final version of their manuscript.
Corrections
If an author wishes to change their name following publication, they can contact the Editorial Office with their request. PJHSL will update and republish the article, with updated metadata provided to indexing services. An Erratum will not be published to protect author identity, and co-authors will not be notified.
Corrections should be submitted for scientifically relevant errors in published articles. Any changes may be evaluated by the academic editors. Any changes that affect the scientific interpretation, such as correcting a misleading portion of a reliable publication, adding missing method details, or errors in data that do not affect conclusions, will be announced using a separate publication called a Correction. The original paper will be updated, and a note will be added to the Article Versions Notes and the abstract page to inform readers of the updated version.
Retractions
Retractions are necessary in cases where articles pose a threat to the integrity of scientific records, such as inadvertent errors, ethical breaches, data fabrication, or plagiarism. PJHSL follows the recommendations of the Committee on Publication Ethics (COPE) for retraction. The Editorial Office investigates potential retractions, with support from the Editorial Board and final approval from the Editor-in-Chief. The article will be amended with a "RETRACTED" watermark but will remain available on the journal's website. Retracted articles should not be cited or used for future research. Retractions are published as a separate item in the current issue of the journal, with the same authorship and affiliation as the original article, and page numbers added. Partial retractions may be published in cases where only some results are incorrect. Complete removal of an article is a rare occurrence, reserved only for cases where leaving it online would be illegal or cause significant harm.
6. PUBLICATION ETHICS AND POLICY GUIDELINES
AUTHORSHIP DISPUTES
Acknowledgements
This journal advocates acknowledging contributions to a scientific work where authorship is not claimed. All additional individuals who contributed to the study and are not a part of authorship should be credited, and their contributions should be detailed, according to ICMJE criteria. All authors must reserve the right to know their contributions were acknowledged along with the technical assistance, data gathering assistance, writing assistance and services offered from departmental head. Support in terms of funding and access to scientific instrumentation should also acknowledged.
Informed consent & maintaining the confidentiality of research participants
- Before including case specifics, other personal information, or images of patients and other persons in their work, writers must first obtain the relevant authorization forms, permits, and releases in order to be in compliance with the country's or region's data protection and privacy laws.
- Each participant who appears in any video, audio, photograph, image, illustration, case report, or other form in which they can be identified, or who is the person's legal guardian, or another person with legal authority to act on their behalf, is informed beforehand that such photographs are being taken or such video, recording, photograph, image, illustration, or report is being made and of all the purposes for which they might be used, including disclosure. That person, their legal guardian, or another person in a position of authority must express their written consent in order for it to be accepted.
- The written consent must abide by all applicable privacy and data protection laws in its entirety. When minors are involved (especially those with special needs or learning difficulties), when an individual's head or face appears, or while the individual's name or other personal information is mentioned, extra caution should be given when acquiring consent.
- In the instance of a child, consent should be regarded not to have been provided and those photographs should not be disseminated if the child's parents or legal guardians disagree with the use of those images. To decrease the probability of images being used improperly, it is also crucial to make sure that only pictures of child wearing appropriate clothing are displayed.
- Even if permission has been granted, care must be taken to ensure that the person being depicted and captioned are courteous and cannot be seen as disparaging that person.
- In order to publish a manuscript, authors must persuade the editors that "informed consent to participate" was obtained from all adult subjects or from the parents or guardians of any minor subjects.
- Names, initials, hospital or social security numbers, dates of birth, or other personal or identifiable information should not be used for patients or research participants.
- Images of patients or research subjects should not be used for publication unless it is absolutely necessary for the scientific investigation and the patient (or parent/guardian) has given written, informed consent. Identifying information ought to be omitted if it is unnecessary, even when consent has been given. Editors may request to submit the written consent.
- In cases where there is any ambiguity regarding anonymity, informed consent is required.
- The authors must make sure that the editors do not misrepresent the scientific meaning while choosing the themes.
- For the use of completely anonymized images from which the individual cannot be identified, such as X-rays, ultrasound images, pathology slides, or laparoscopic images, formal consent is not necessary so long as the images are free of any identifying marks and are not accompanied by text that could place the individual at risk of being identified.
- It is typically insufficient to anonymize a photo simply by adding eye bars or obscuring the subject's face if authorization has not been obtained.
- The CARE case report writing guidelines should be followed by authors.
Conflicts of interest/ Grant support and financial disclosure
- Any conflicts of interest including financial or personal ties to other people, businesses, or organizations that can improperly affect (bias) their work must be disclosed by all authors.
- Financial conflicts include, but are not limited to, work-related consultancy, stock ownership, honoraria, expert testimony that was paid for, patents or patent applications, and travel grants; all of which started within three years of the work that was submitted.
- Authors should declare no conflict of interest.
- Authors of studies who have received funding from an organization are required to sign a statement attesting to their full access to all the study's data and their full responsibility for the validity of the data they gathered and the reliability of their data analysis. This declaration must be included with the manuscript.
- A further obligation for reviewers is to disclose any conflicts they may have with the work they are assigned to assess.
- This journal publishes conflicts of interest/ grant support and financial disclosure along with all published items.
PROTECTION OF RESEARCH PARTICIPANTS
Approval of Ethical Committee/ Use of humans and animals/ Helsinki Declaration
- We presume that the authors believe in the culture of responsible research.
- In order to uphold the fundamental principles of research objectivity, honesty, openness, fairness, accountability, and stewardship we encourage research integrity.
- We support the fundamental principles of the COPE standards and handle any alleged conduct in accordance with them.
- At the time the article is submitted, the ethical approval certificate for any research involving individuals or animals must be provided.
- The most recent version of the Helsinki Declaration must be used and noted when reporting studies involving human participants.
- For medical research involving human subjects, we abide by the ICMJE Recommendations on protection of research participants and the Declaration of Helsinki-related ethical principles of the World Medical Association (WMA)
- If there is any question about whether the study was carried out in line with the Helsinki Declaration, the authors must justify their strategy and show that the institutional review board specifically accepted any questionable components of the trial.
- The WMA declaration on animal use in biomedical research, the consensus author guidelines on animal ethics and welfare from the International Association of Veterinary Editors, and the guide for the care and use of laboratory animals should all be followed by authors.
- Authors must certify in writing that all legal and ethical regulations for the human treatment of the animals used in the study have been complied with.
- The relevant steps used to reduce pain or discomfort should be noted in the Methods (experimental methods) section, and information regarding animal care should be included.
- This journal suggests the following 3Rs principal for using people and animals in research.
Replacement: approaches, which avoid or replace the use of animals.
Reduction: approaches, which minimize the number of animals used per experiment.
Refinement: approaches which minimize animal suffering and improve welfare.
Research Ethics Policies for Studies Involving Clinical Trials (CT) Registration
This policy aligns with the criteria set forth by the International Committee of Medical Journal Editors (ICMJE). The ICMJE defines a clinical trial as a research project that assigns human subjects to intervention or comparison groups in a prospective manner to investigate the causal relationship between a medical intervention and a health outcome. Studies with different objectives, such as examining pharmacokinetics or significant toxicity (e.g., phase 1 trials), would be exempt from this definition.
While the ICMJE doesn't endorse a specific registry, authors submitting to the Pakistan Journal of Health Sciences are required to register their trials in an international trial registry system that meets specific criteria.
This registry should be accessible to the public without any charges, open to all potential registrants, and managed by an established organization.
It is essential for the registration data to be validated, and the registry itself should be searchable electronically.
A comprehensive registry should encompass the following details at a minimum: a unique identification number, a description of the interventions and comparisons under study, the study hypothesis, definitions of primary and secondary outcome measures, eligibility criteria, crucial trial dates (registration, anticipated or actual start, last follow-up, closure to data entry, and completion date), the expected number of participants, funding source, and contact information for the principal investigator.
Registration is just one facet of achieving complete transparency regarding the execution and reporting of clinical trials.
Participants who volunteer for clinical trials should be informed that their valuable contribution to advancing human health will be accessible to guide healthcare decisions. The collective generosity of these individuals should result in knowledge that's accessible to everyone. Mandatory trial registration serves as a step toward this objective. The Clinical Trial Registration number must be included in the manuscript and clearly stated.
7. PLAGIARISM POLICY
Plagiarism is the presenting of another author's language, text, thoughts, ideas, or expressions as one's own unique work. Self-plagiarism is also included, which includes duplicate/redundant publication, content recycling, and salami slicing. PJHSL adheres to the COPE, ICMJE, and HEC (Higher Education Commission) of Pakistan recommendations, norms, and policies on plagiarism. PJHSL uses TURNITIN to check the similarity index, and notifications are sent to the authors if it is greater than 50%. If it is greater than 50%, it will be rejected without further inspection or processing. For authenticity, the Higher Education Commission (HEC) of Pakistan needs a similarity of less than 20%. If the authors have already checked the paper with the same software, they can submit the similarity report as a supplementary file. Plagiarism is considered academic dishonesty. If it is proven after the article has been published, it will be retracted, the authors may be permanently or temporarily blocked, and the parent institution may be notified for departmental proceedings against the author. If it is proven before publication, the present work will be rejected, and the authors may be prohibited from submitting to this journal for one or more years.
8. POLICIES RELATED TO THE USE OF AI IN MANUSCRIPTS
We recognize the growing role of Artificial Intelligence (AI) in various aspects of research, including manuscript preparation. To ensure transparency, integrity, and proper attribution in scholarly publications, the following policy regarding the use of AI-assisted technologies in manuscript preparation is hereby established:
Disclosure Requirement: Authors utilizing AI-assisted technologies such as chatbots or image creators in the preparation of their manuscripts are required to disclose this information explicitly in their submissions. This disclosure should be included in the manuscript's methodology section or in a separate statement provided by the authors.
Non-Attribution of AI as an Author: AI algorithms, tools, or systems should not be listed as authors of the manuscript. The authorship of the paper should accurately reflect the contributions of human researchers involved in the study, analysis, and interpretation of results.
Human Oversight and Responsibility: While AI may play a significant role in generating content, humans remain responsible for overseeing the entire manuscript preparation process. Authors are expected to ensure the appropriateness and accuracy of AI-generated content, as well as to verify the absence of plagiarism or any ethical concerns related to the use of AI.
Attribution of AI-generated Content: Proper attribution should be provided for any content generated or substantially influenced by AI technologies. Authors must clearly indicate in the manuscript which sections or elements were created with the assistance of AI tools, along with appropriate citations or references to the relevant algorithms, datasets, or software used.
9. REPOSITORY POLICY
Authors are permitted to deposit all versions of their paper in an institutional or subject repository:
1. Preprint
2. Author’s Accepted Manuscript
3. Version of Record
No embargo is applied.
10. DATA SHARING POLICY
Authors can submit their data in any public repository and share the information or link under this section at the end of the manuscript.
11. ADVERTISEMENT POLICY
All advertisements are subject to the approval of the PJHSL Management, which reserves the right to reject or cancel any ad at any time.
All advertisements are accepted and published by PJHSL on the warranty of the agency and advertiser that both are authorized to publish the entire contents and subject matter of the advertisement.
In consideration of publication of an advertisement, the advertiser and the agency, jointly and severally, agree to indemnify and hold harmless Publisher, its officers, agents and employees against expenses (including legal fees) and losses resulting from the publication of the contents of the advertisement, including, without limitation, claims or suits for libel, violation of privacy, copyright infringement, or plagiarism.
PJHSL will not be liable for any failure to publish any advertisement accepted by PJHSL; however, PJHSL shall use its reasonable efforts to place such advertisement in subsequent available space.
All advertisements must clearly and prominently identify the advertiser by trademark or signature.
Any references to PJHSL or its products or services in advertisements, promotional material, or merchandising by the advertiser or agency is subject to PJHSL’s written approval for such use.
All advertising contract position clauses are treated as requests. PJHSL cannot guarantee fixed positioning.
PJHSL is not responsible for incidental or consequential damage for errors in displaying or printing an ad.
PJHSL may change the terms set forth herein at any time, provided that no such change applies to ads whose closing date precedes the announcement of the change.
PJHSL will not be bound by any condition, printed or otherwise, appearing on any insertion order or copy instructions.
In the event of nonpayment, PJHSL reserves the right to hold advertiser and/or its advertising agency jointly and severally liable for such monies as are past due and payable to PJHSL.
Proprietary names of pharmaceutical products must be accompanied by the chemical, generic, or official name; the quantity of all active substances must be stated along with the recommended dosage. New ad copies and creative for pharmaceutical products should be sent to the advertising department. Please allow two weeks for clearance.
Advertiser represents and warrants that all advertisements and pharmaceutical products they advertise are compliant with all applicable laws, rules, and regulations in the country where the advertisement will be seen. Advertisements for pharmaceutical products must comply with Concerned Government Department regulations regarding advertising and promotion.
DIGITAL ADS: Any use of PJHSL trademarks or copyrighted material for links to and from PJHSL’s website must be approved in advance by PJHSL. Any unauthorized linking is prohibited. PJHSL does not endorse or support any product or organization linked to its website, nor is PJHSL responsible for the content of any website promoted in an ad. The use by advertiser or its agency of pixels, beacons, cookies, tracking tags, or similar technology in advertising creative for the purpose of collecting personally identifiable information is prohibited.
Digital Cancellation Policies
Advertiser may cancel the entire insertion Order, or any portion thereof, as follows:
RUN-OF-SITE BANNER PROGRAMS
On written notice to the PJHSL, advertiser may cancel all, or a portion of the campaign, without penalty 21 days or more before the campaign start date. For cancellations made within 21 days of the start date, the advertiser will be responsible for 50% of the campaign amount that was reserved for delivery.
FLAT FEE-BASED, SOV-BASED, OR FIXED PLACEMENT PROGRAMS
On written notice to the Publisher, advertiser may cancel all, or a portion of the campaign, without penalty 30 days or more before the start date of the campaign. For cancellations made 30 to 15 days before the start date, advertiser will be responsible for 50% of the campaign amount that was reserved for delivery. For cancellations made within 14 days of the start date, advertiser will be responsible for 100% of the campaign amount that was reserved for delivery.
12. APPEALS PROCESS
The readers, authors, reviewers or any other person may submit a formal appeal through official email (editor@thejas.com.pk) of the journal regarding any problem, including but not limited to any conflict, delays in review or publishing or article processing charges or rejection of manuscripts to the Chief Editor. The case will be referred for examination/ investigation to the Appeals Committee of the Editorial Board/ Advisory Board to give recommendations to the Board for decision. The Committee is comprised of;
- Dr. Asif Nadeem
- Dr. Aditya Mojumdar
- Dr. Shahbaz Ahmad Zakk
- COMPLAINTS PROCESS
Regarding any publication misconduct on the part of an author, a reviewer, or the Editor/Editorial Board, readers, writers, or any other person may send a written complaint to the Chief Editor using the journal's official email address (editor@thejas.com.pk). The Complaints Process Committee of the Editorial Board/Advisory Board will be asked to investigate the issue and make suggestions to the Board regarding its course of action. The Committee is comprised of;
- Dr. Riffat Mehboob
- Dr. Muhammad Ayaz Anwar
- Dr. Rizwan Ullah Khan
14. SUBMISSION OF FALSE DOCUMENTS
Authors are expected to submit all necessary documents accurately and completely during the submission process.
While the journal does not assume direct liability for false authorship letter/ethical approval letters/data collection permission/trial registration number submitted by authors, it is committed to upholding ethical standards and ensuring the integrity of published research. Upon suspicion or discovery of false documentation, the journal reserves the right to verify the authenticity of all submitted documents, including but not limited to institutional review board (IRB) letters, ethical approval letters, and authorship declarations. Verification procedures may include contacting relevant institutions or authorities, cross-referencing with publicly available databases, or seeking clarification from the authors. Authors are expected to cooperate fully with the verification process and provide any requested information or documentation promptly and accurately. Failure to cooperate with verification procedures may result in rejection of the manuscript or other disciplinary actions.
Authors found to have submitted false documentation may be subject to sanctions, including but not limited to:
Blacklisting from submitting manuscripts to the journal for a minimum period of three (3) years.
Referral to relevant professional bodies or regulatory authorities for further investigation and potential disciplinary action.
COPYRIGHT NOTICE
This work is licensed under a Creative Commons Attribution License.
Readers may “Share-copy and redistribute the material in any medium or format” and “Adapt-remix, transform, and build upon the material”. The readers must give appropriate credit to the source of the material and indicate if changes were made to the material. Readers may not use the material for commercial purpose. The readers may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
PRIVACY STATEMENT
The names and email addresses entered in this journal site will be used exclusively for the stated purposes of this journal and will not be made available for any other purpose or to any other party.
AUTHOR FEES
Article Processing Fee: Rs 5000/-
Article Publication Fee (National) Rs 30000 / Article
Article Publication Fee (International ) 200 USD / Article
Waivers Policy:
If an author has no funds to pay such charges, he may request for full or partial waiver off of publication fees. We do not want charges to prevent the publication of worthy work.
Hard Copies:
Copies can be ordered on payment of PKR 2500 per copy.








